Abstract
Introduction: Chronic lymphocytic leukemia (CLL) is the most common leukemia in adults in the United States (US) with an estimated 18,960 new cases for 2016 [ American Cancer Society. Cancer Facts & Figures 2016 ]. CLL is a disease of the elderly; the median age at diagnosis in the US is 71 years, and most patients do not reach criteria for treatment until years after their diagnosis [ SEER Stat Fact Sheets: Chronic Lymphocytic Leukemia ]. Several front-line combination therapies and monotherapies are approved for the treatment of CLL; however, few studies have examined the prevalence of their use in the real world or differences in patient characteristics across regimens. Thus, the current analysis characterizes the real-world prevalence of CLL front-line regimens, as well as patient characteristics across front-line regimens.
Methods: CLL patients (ICD-9: 204.1x or ICD-10: C91.1x, C83.0x) receiving front-line therapy between 11/01/2008 and 5/31/2017 were identified in the Flatiron Health electronic health records database [ Flatiron Health Data, New York, NY (5, 31,2017)] . Differences among cohorts were examined using general linear modeling for normally distributed variables and non-parametric tests (ie, chi-square and Kruskal Wallis) for non-normally distributed variables.
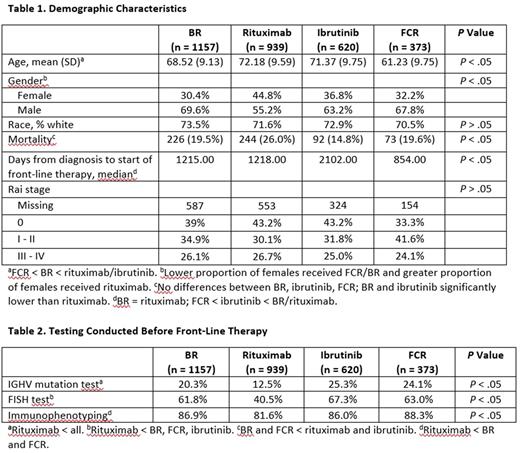
Results: A total of 38 different front-line therapies in 5124 patients were identified. The top four regimens received by 3089 of the patients included: bendamustine plus rituximab (BR; n = 1157); rituximab monotherapy (n = 939); ibrutinib monotherapy (n = 620); and fludarabine, cyclophosphamide, and rituximab (FCR; n = 373). The mean age at the time of treatment of the sample was 69.32 years (SD = 10.06; median = 70) and males comprised 64% of the sample. The BR (68.52 years; SD = 9.13) and FCR (61.23 years; SD = 9.75) cohorts were significantly younger than the rituximab (72.18 years; SD = 9.59) or ibrutinib (71.37 years; SD = 9.75) cohorts. A significantly lower proportion of females relative to males received BR, FCR, or ibrutinib, compared with the cohort who received rituximab. No differences in overall mortality were observed among the BR, ibrutinib, or FCR cohorts; however, both the BR and ibrutinib cohorts had significantly (P < .05) lower mortality rates than the rituximab cohort. Patients in the FCR began therapy significantly sooner after diagnosis relative to the BR, rituximab, and ibrutinib cohorts. No differences in severity based on Rai stage were found. IGHV analysis was conducted in less than 25% of the sample, and fluorescence in situ hybridization (FISH) testing was conducted in approximately 60% of the sample. Patients in the rituximab cohort received less mutational testing, FISH testing, and immunophenotyping prior to starting therapy.
Conclusions: Results from long term real-world data suggest that while several front-line CLL therapies exist, the majority of patients during the time period of this study (2008-2017) were treated with one of four regimens: BR, rituximab monotherapy, ibrutinib, or FCR. Patients receiving FCR and BR are significantly younger compared with those receiving rituximab monotherapy, and FCR patients were significantly younger than BR patients. CLL has a known male predominance [ American Cancer Society. Cancer Facts & Figures 2016 ], but notably, there was a significant male/female difference identified in this study in selection of front-line therapy, with a lower proportion of females starting therapy with FCR or ibrutinib and a greater proportion starting on rituximab. Older age, higher mortality rate, and less IGHV and FISH testing were observed in the rituximab monotherapy cohort, suggesting that this cohort may have included less fit patients. Low rates of IGHV mutational status and FISH testing in these front-line patients is in line with other published reports [ eg, Mato A, et al. Br J Haematol 2016;175:892-903 ], and may fail to identify favorable-risk patients who can have extended remissions with chemoimmunotherapy, as well as high-risk patients who benefit from novel therapeutic approaches.
Tang: Teva Pharmaceuticals: Employment. Szabo: Teva Pharmaceuticals: Employment. Brander: Teva Pharmaceuticals, Genentech, AbbVie, Pharmacyclics: Consultancy; Genentech: Consultancy; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal